Children aged 3 through 17 years
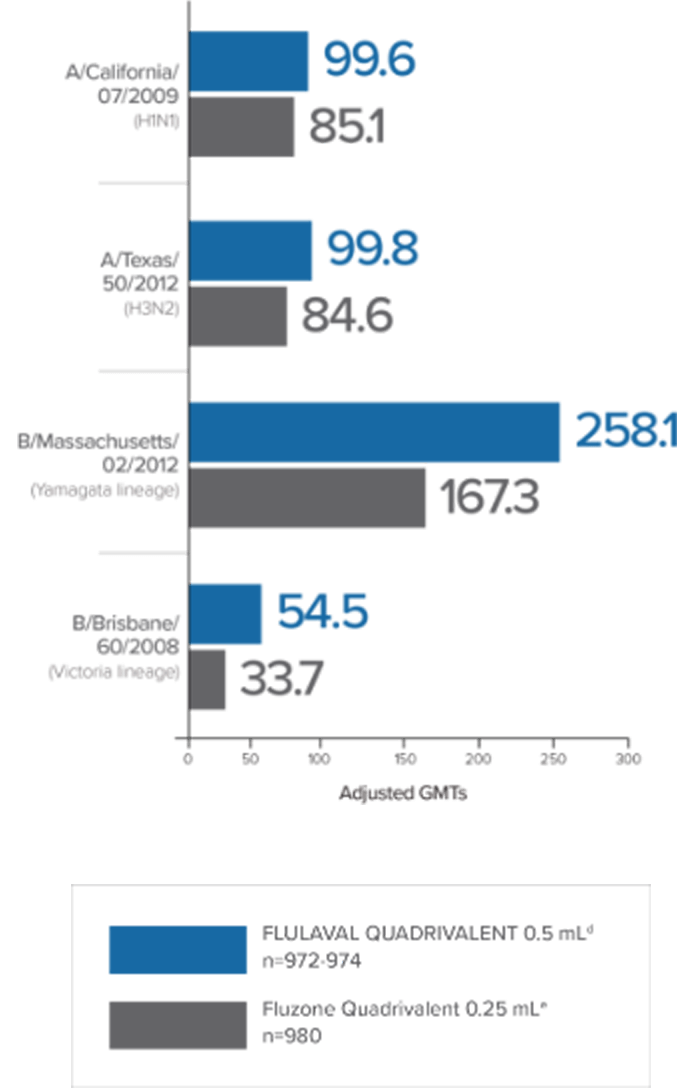
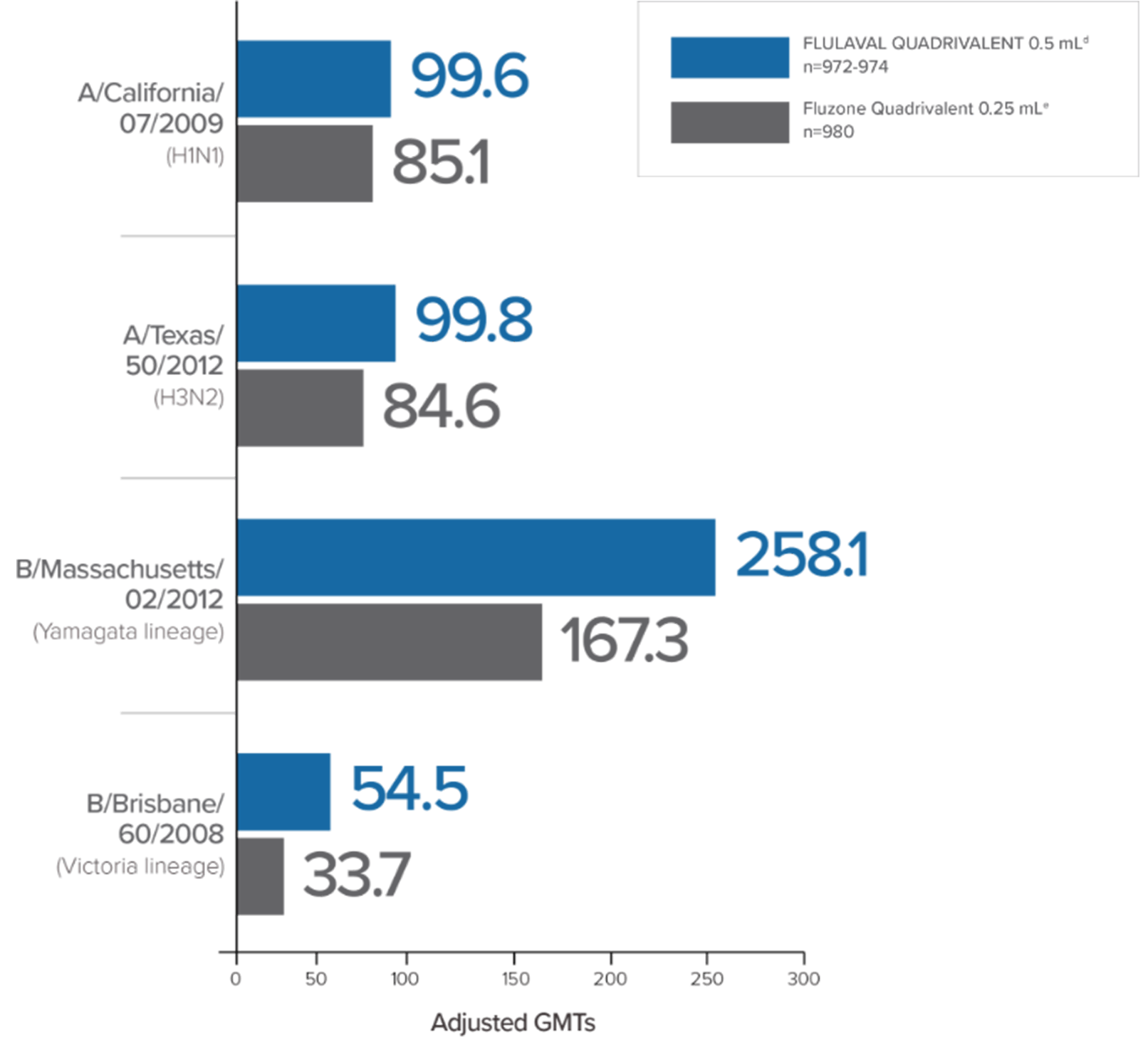
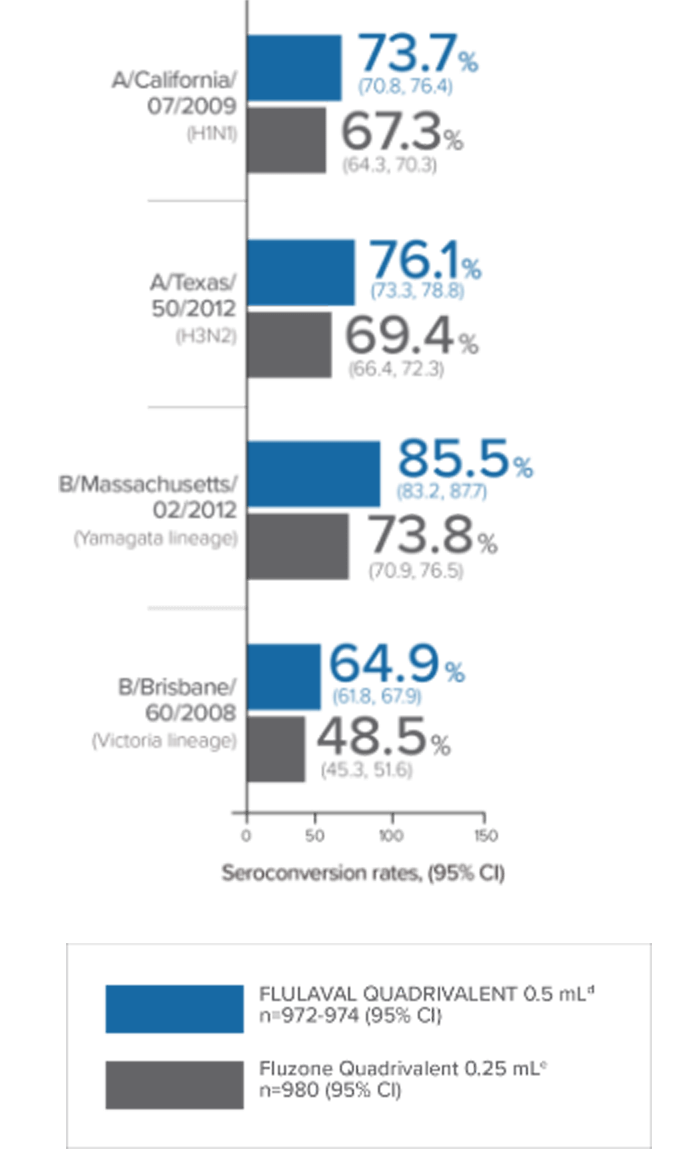
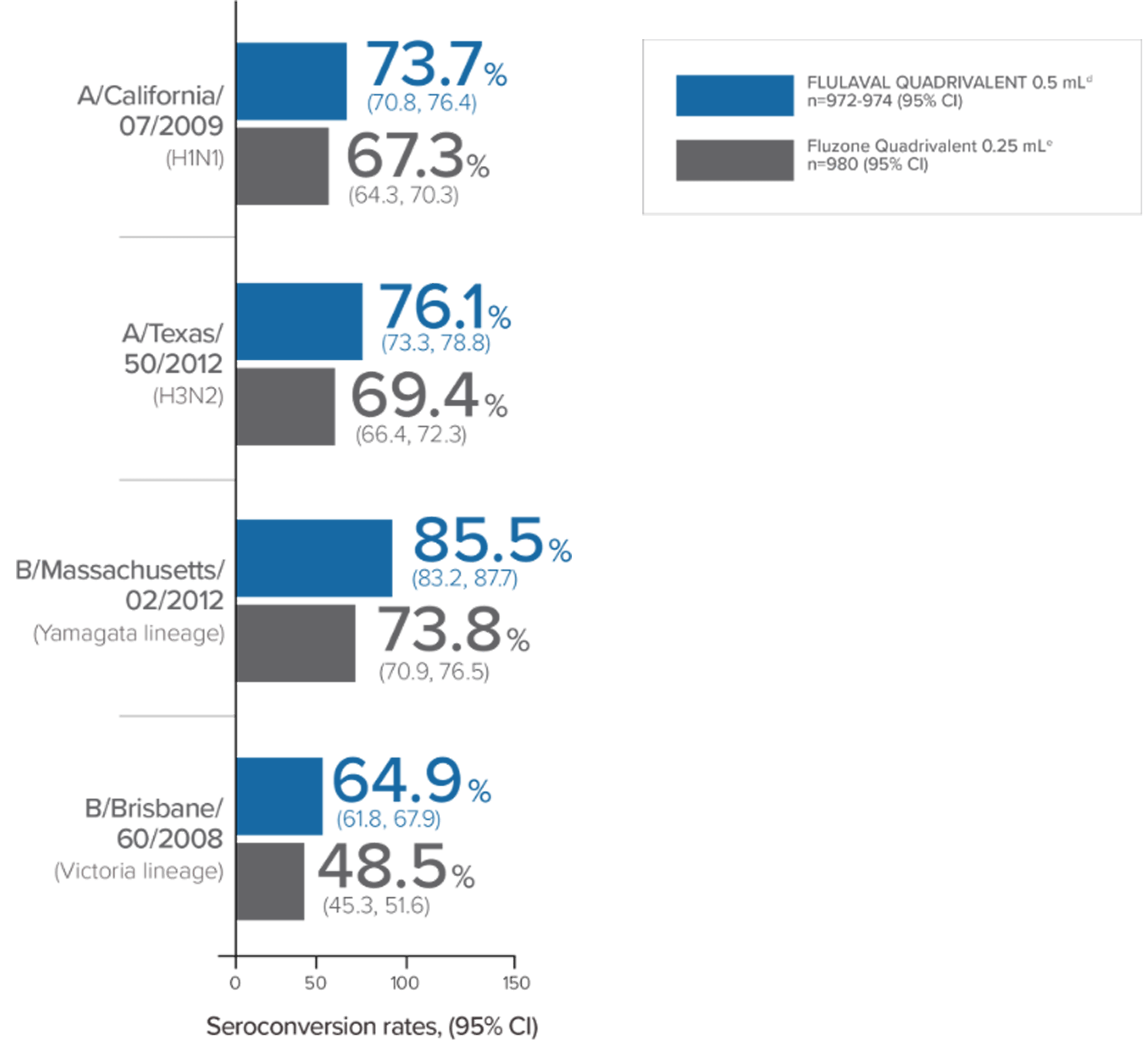
In a clinical trial in subjects aged 3 through 17 years, FLULAVAL QUADRIVALENT (n=878) exhibited immunogenicity noninferior to two comparator trivalent inactivated influenza vaccine (TIV) formulations, each containing an influenza type B virus that corresponded to one of the two B viruses in FLULAVAL QUADRIVALENT, a type B virus of the Victoria lineage (TIV-1, n=871) or a type B virus of the Yamagata lineage (TIV-2, n=878).
- Noninferiority was based on adjusted GMTs and seroconversion rates1
- Seroconversion was defined as a 4-fold increase over baseline in post-vaccination hemagglutination inhibition (HI) antibody titers from pre-vaccination titer ≥1:10, or an increase in titer from <1:10 to ≥1:401